药品详情:
【奥氮平片Zyprexa 适应症】
奥氮平片,适应症为奥氮平用于治疗精神分裂症。初始治疗有效的患者,奥氮平在维持治疗期间能够保持基临床效果。奥氮平用于治疗、重度躁狂发作。对奥氮平治疗有效的躁狂发作患者,奥氮平可用于预防双相情感障碍的复发。
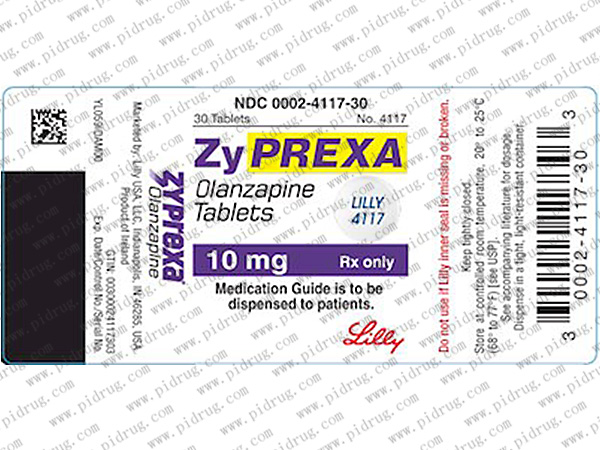
【奥氮平片Zyprexa 规格】
本品为片剂,每片含有效成份2.5mg/5mg/7.5mg/10mg/15mg/20mg等多种规格,每瓶30片装。
【奥氮平片Zyprexa 服用方法】
精神分裂症:
奥氮平的建议起始剂量为10mg/天,每日一次,与进食无关。
在精神分裂症的治疗过程中,可以根据患者的临床状态调整日剂量为5~20mg/天,建议经过适当的临床评估后,剂量加增加到10mg/天的常规剂量以上,加药间隔不少于24小时。停用奥氮平时应逐渐减少剂量。
躁狂发作:
单独用药时起始剂量为每日15mg,合并治疗时每日10mg
预防双相情感障碍复发:
推荐起始剂量为10mg/天,对于使用奥氮平治疗躁狂发作的患者,预防复发的持续治疗剂量同前。对于新发躁狂、混合发作或抑郁发作,应继续奥氮平治疗(需要时剂量适当调整),同时根据临床情况合并辅助药物治疗情感症状。
在精神分裂症、躁狂发作和双相情感障碍的预防治疗过程中,可根据个体临床状况不同,在5-20mg/日的范围内相应调整每日剂量,建议仅在适当的临床再评估后方可使用超过推荐剂量的药物,且加药间隔不少于24小时,奥氮平给药不用考虑进食因素,食物不影响吸收,停用奥氮平时应逐渐减少剂量。
肾脏和/或肝脏功能损害的患者:
对这类患者应考虑使用较低的起始剂量(5mg)。中度肝功能不全(肝硬变、Child-pugh分级为A或B级)的患者初级剂量为5mg,并应慎重加量。
女性患者与男性相比:
女性患者的起始剂量和剂量范围一般无须调整。
非吸烟患者与吸烟患者相比:
非吸烟患者的初始剂量和剂量范围一般无须调整。
当有不止一个减缓代谢的因素(女性、年老、非吸烟的)出现时,应考虑降低起始剂量,需要增加剂量时也应该保守。
【奥氮平片Zyprexa 注意事项】
罕有高血糖的报道,有糖尿病史的患者会罕见酮症酸中毒或昏迷,亦有数例死亡病例报道。某些病例报道有既往的体重增加,这可能是一种促发因素,建议对糖尿病人和存在糖尿病高危因素的人进行适当的临床监查。
突然停用奥氮平时,极少出现下列急性症状,诸如出汗、失眠、震颤、焦虑、恶心或呕吐等(<0.01%)。停用奥氮平时建议逐渐减量。
合并症:离体实验证明奥氮平具有抗胆碱能活性,但临床试验中发生的与抗胆碱作用相关的事件很低。然而,奥氮平治疗有合并疾病的患者的临床经验有限,建议奥氮平慎用于前列腺肥大或麻痹性肠梗阻以及相关病症的患者。
不推荐使用奥氮平治疗帕金森病及与多巴胺激动剂相关的精神病。在临床试验中,有报道这类患者服用奥氮平后帕金森症状恶化,或幻觉比安慰剂更为常见和频繁(参见不良反应),而奥氮平对于这些患者的精神病性症状的疗效与安慰剂相当。在这些试验中,要求患者使用最低起始有效剂量的抗帕金森药物(多巴胺激动剂)保持稳定状态,并且在整个试验过程中保持使用的抗帕金森药物种类和剂量的一致。奥氮平起始为2.5mg/日,并根据研究者的判断最高调整到15mg/日。
奥氮平没有被批准用作治疗痴呆有关的精神病和/或行为紊乱,对这类特殊的患者也不推荐使用,因为有增加死亡率和脑血管事件的风险。在一项安慰剂对照的临床试验中(6-12周),受试者为患有痴呆的精神病和/或行为紊乱的老年人(平均年龄78岁)。和安慰剂比较,用奥氮平治疗的患者的死亡率有2倍的增加(分别为3.5%,1.5%)。但死亡发生率与奥氮平的剂量(平均日剂量为4.4mg)或治疗的周期无正相关性。导致死亡率升高的风险因素包括,年龄大于65岁,吞咽困难,镇静状态,营养不良和脱水,肺部疾病(如吸入或非吸入性肺炎),或同时服用苯二氮卓。然而,排除这些风险因素,使用奥氮平治疗的患者的死亡率依然高于服用安慰剂的患者。
在同一临床研究中,有报道脑血管不良事件(CVAE,即中风,瞬时的缺血发作),其中包括死亡病例。用奥氮平治疗的患者出现脑血管不良事件的发生率为安慰剂的3倍(分别是1.3%,0.4%)。所有出现脑血管不良事件的用奥氮平和安慰剂治疗的患者均有已经存在的风险因素。与奥氮平治疗有关的CVAE的风险因素包括年龄大于75岁和血管/混合型痴呆。奥氮平的有效性在这些试验中没有证明。
在治疗精神病的过程中,患者临床状况的好转可能需要几天甚至几个星期。在此期间应密切监护患者。
乳糖:奥氮平片剂中含有乳糖。
患者服药期间常会出现短暂的无症状性的肝脏转氨酶ALT、AST升高,尤其是治疗早期。因此ALT和/或AST升高的患者、有肝功能损害症状或体征的患者、已表现出局限性肝脏功能减退的患者以及已使用潜在肝毒性药物治疗的患者应慎用奥氮平。治疗期间如出现ALT和/或AST升高,应注意观察并考虑酌减用药量。在业已诊断有肝炎的情况下,应该中断奥氮平治疗。上市后很少接到肝炎的报告,以及极少接到胆汁阻塞或混合性肝损伤的报告。
脂质改变:在安慰剂对照的临床试验中发现接受奥氮平治疗的患者发生不良的脂质改变(参见【不良反应】),建议进行适当的临床监测。
心血管死亡:在一项回顾性观察研究中,与未服用抗精神病药物的患者相比,使用非典型抗精神病药物(包括奥氮平)或典型抗精神病药物治疗的患者均存在推定心脏性猝死风险的升高,且均与剂量相关(后者风险几乎是未服用抗精神病药物患者的两倍)。在奥氮平的上市后报告中,心脏性猝死事件的报告非常罕见。
与其他神经阻滞剂类似,奥氮平慎用于白细胞和/或中性粒细胞计数减低的患者,服用已知能引起中性粒细胞减少症的患者,有药物所致的骨髓抑制/毒性作用病史的患者,合并疾病、放疗或化疗导致骨髓抑制的患者以及嗜酸细胞增多症或骨髓增生症的患者。32名有与氯氮平相关的中性粒细胞减少或粒细胞缺乏病史的患者在奥氮平治疗后未发生中性粒细胞减低,奥氮平与丙戊酸钠合并使用时常见中性粒细胞减少症。
有关合并使用锂盐和丙戊酸钠的资料有限。尚无奥氮平与卡马西平合并使用的临床资料,只进行过药代动力学研究。
神经阻滞剂恶性综合征(NMS):NMS是一种与抗精神病药物有关的潜在致死性的疾病。用奥氮平治疗的患者罕有NMS的报道。NMS的临床特征是高热、肌强直、意识改变和植物神经系统功能不稳定(脉搏和血压不规则、心动过速、大汗以及心脏节律紊乱)。附加症状还包括肌酸磷酸激酶升高、肌红蛋白尿(横纹肌溶解)以及急性肾衰。如果患者的症状和体征提示NMS,或表现为不能解释的高热而不伴有NMS的其他临床特征,那么所有的抗精神病药物,包括奥氮平均应停用。
奥氮平慎用于有惊厥发作史和有惊厥阈值降低因素的患者。目前奥氮平引起惊厥的报道很少,这些病例绝大多数报告有惊厥史和惊厥危险因素。
迟发性运动障碍:在为期一年或更短的对照研究中,奥氮平治疗中发生的运动障碍较少,且有统计学显著性。但长期用药会使迟发性运动障碍的危险性增加。因此,若用奥氮平治疗的患者出现迟发性运动障碍的症状和体征,应考虑减少用药量或停药。停止治疗后这些症状可能会出现一过性恶化甚或加重。
考虑到奥氮平对中枢神经系统的基本作用,与其他中枢活性药物合用时或用于饮酒患者时应慎重。由于离体奥氮平表现出多巴胺拮抗作用,故可能拮抗直接或间接的多巴胺激动剂的作用。
奥氮平治疗老年患者的临床实验中,偶有体位性低血压的报道。与其他抗精神病药一样,用奥氮平治疗65岁以上的患者时建议定期监测患者的血压。
临床试验中,接受奥氮平治疗的患者出现有临床意义的QTc间期延长(基线QTcF500毫秒)并不常见(0.1%-1%),和安慰剂相比,没有统计学差异。但与其他抗精神病药一样,奥氮平与其他已知可以延长QTc间期的药物合用时要谨慎,尤其是在老年患者、先天性长QT综合征患者、充血性心脏衰竭患者、心肌肥厚、低钾血症或低镁血症的患者。
对奥氮平治疗与出现静脉栓塞之间的瞬时联系罕有报道(<0.01%),两者之间的联系尚未确认。然而,由于精神分裂症患者往往伴有后天静脉栓塞的风险,因此所有可能与静脉栓塞相关的风险因素(如对患者实施固定术)均应给予考虑,并采取预防措施。
由于奥氮平可能导致瞌睡,患者在操作危险性机械包括机动车时应格外小心。
【奥氮平片Zyprexa 不良反应】
成人
体重
在临床试验中,奥氮平治疗的患者体重均值增加大于安慰剂治疗组的患者。所有基线体重指数(BMI)分类中均观察到临床显著的体重增加。
在长期临床试验(至少48周)中,体重增加程度和奥氮平治疗组病人体重临床显著性增加的比例均高于短期临床试验。长期用药时体重增加超过25%基线体重的病人百分率(≥10%),很常见。
葡萄糖
在临床试验(52周)中,相对于安慰剂组而言,奥氮平组,葡萄糖均值变化较大。
奥氮平与安慰剂对比,葡萄糖均值变化在伴有基线葡萄糖失调证据的患者中增大(包括那些诊断为糖尿病的患者或符合高血糖标准的患者),这些患者对比安慰剂治疗患者糖化血红蛋白(HbA1c)增加更大。
血糖变化从正常或临界基线水平增加到高水平的病人比例随时间增加。在一项完成奥氮平治疗9-12个月病人的分析中,约6个月后平均血糖增长率减慢。
血脂
在为期12周的临床试验中,与安慰剂治疗组患者比较,奥氮平治疗的患者空腹总胆固醇、LDL胆固醇和甘油三酯浓度均值增加更大。
没有基线血脂失调证据的患者空腹脂值(总胆固醇、LDL胆固醇和甘油三酯)更大。
关于空腹HDL胆固醇,奥氮平治疗患者与安慰剂治疗患者之间未观察到统计学显著差异。
在长期临床试验(至少48周)中,总胆固醇、LDL胆固醇或甘油三酯变化从正常或临界水平变化到高水平的病人比例,或HDL胆固醇变化从正常或临界水平变化到低水平的病人比例,均大于短期临床试验。在一项完成12个月治疗的病人分析中,约4-6个月后平均非空腹总胆固醇没有进一步增加。
催乳素
在一项对照临床试验中(长达12周),相比于安慰剂组10.5%的患者催乳素升高,奥氮平治疗组30%的患者催乳素升高,绝大多数患者为轻度。精神分裂症患者的催乳素水平随着治疗的持续而下降,与催乳素升高1相关的月经方面的不良事件较常见(发生率<10%,≥1%),而性功能及乳房方面的不良事件不常见(发生率<1%,≥0.1%)。其他精神疾病2患者的催乳素水平随治疗的继续而持续升高,与催乳素相关的性功能方面的不良事件较常见(发生率<10%,≥1%),而乳房及月经方面的不良事件不常见(发生率<1%,≥0.1%)。
(1)TESEs分析长达52周治疗。
(2)双相抑郁,精神病性抑郁,难治性抑郁,边缘型人格障碍和双相躁狂。
肝脏转氨酶
偶见无症状暂时性肝脏转氨酶升高,ALT/SGPT和AST/SGOT。
嗜酸性粒细胞增多
偶见无症状的嗜酸性粒细胞增多。
特殊群体的不良反应:在痴呆性老年精神病患者进行的临床试验中,与奥氮平治疗相关的很常见(≥10%)不良反应是异常步态和跌倒。在痴呆性老年精神病患者进行的临床试验中,与奥氮平治疗相关的常见(<10%且≥1%,不良反应是尿失禁和肺炎。在与帕金森病相关的药物(多巴胺激动剂)诱导的精神病患者的临床试验中,帕金森症状加重的报告很常见,比安慰剂组频率高。幻觉报告也很常见,也比安慰剂组频率高。在这些临床试验中,要求患者开始研究前服用固定最小剂量的抗帕金森病药物(多巴胺激动剂),并在整个研究过程中维持该剂量不变。奥氮平初始剂量2.5mg/日,根据研究者的判断逐渐增加剂量,最大剂量15mg/日。
青少年(13-17岁)
在奥氮平治疗的青少年患者中,观察到的不良反应类型与奥氮平治疗成年患者中观察到的类型相似。尽管没有进行青少年和成人的对比临床试验设计,但还是比较了青少年临床试验数据和成人临床试验数据。
青少年(疗程中位数3周,体重增加4.6kg)体重平均增加大于成年(疗程中位数7周体重增加2.6kg)。
在长期临床试验(至少24周))中,体重增加程度和奥氮平治疗组青少年病人体重临床显著性增加的比例均高于短期临床试验及成人组病人。长期用药,约一半青少年病人体重增加超过15%基线体重和约三分之一的青少年病人体重增加超过25%基线体重。在青少年病人中,平均体重增加在超重或基线肥胖病人中最为明显。
奥氮平治疗的青少年患者和成人患者比较,空腹血糖水平的增加相似;然而,与成人患者比较,青少年奥氮平组与安慰剂组之间的差异更大。
在长期临床试验(至少24周)中,血糖变化从正常基线水平变化到高水平不常见(发生率0.1%—1%)。
奥氮平治疗的青少年与成人比较,空腹总胆固醇水平、LDL胆固醇水平和甘油三酯水平的增加通常更大;然而,在短期临床试验中,奥氮平与安慰剂组间的差异在青少年患者和成人患者是相似的。
与成人相比,奥氮平治疗引起的青少年患者的催乳素升高发生率更高,催乳素水平升高的平均值更大。
奥氮平片Zyprexa(Olanzapine)
Olanzapine, sold under the trade name Zyprexa among others, is an atypical antipsychotic primarily used to treat schizophrenia and bipolar disorder.[7] For schizophrenia, it can be used for both new onset disease and long term maintenance.[7] It is taken by mouth or by injection into a muscle.[7]
Common side effect include weight gain, movement disorders, dizziness, feeling tired, constipation, and dry mouth.[7] Other side effects include low blood pressure with standing, allergic reactions, neuroleptic malignant syndrome, high blood sugar, seizures, gynecomastia, and tardive dyskinesia.[7] In older people with dementia, its use increases the risk of death.[7] Use in the later part of pregnancy may result in a movement disorder in the baby for some time after birth.[7] Although how it works is not entirely clear, it blocks dopamine and serotonin receptors.[7] It is classed as an atypical antipsychotic.[7]
Olanzapine was patented in 1971 and approved for medical use in the United States in 1996.[8][7] It is available as a generic medication.[7] In the United States, the wholesale cost is less than US$0.25 per dose as of 2018.[9] In 2016, it was prescribed more than 2 million times in the United States.[10]
Medical uses
Schizophrenia
The first-line psychiatric treatment for schizophrenia is antipsychotic medication; with olanzapine being one such medication.[11] Olanzapine appears to be effective in reducing symptoms of schizophrenia, treating acute exacerbations, and treating early-onset schizophrenia.[12][13][14][15] The usefulness of maintenance therapy, however, is difficult to determine as more than half of people in trials quit before the six-week completion date.[16]Treatment with olanzapine (like clozapine) may result in increased weight gain and increased glucose and cholesterol levels when compared to most other second-generation antipsychotic drugs used to treat schizophrenia.[13][17]
Comparison
National Institute for Health and Care Excellence, the British Association for Psychopharmacology, and the World Federation of Societies for Biological Psychiatry suggest that there is little difference in effectiveness between antipsychotics in prevention of relapse, and recommend that the specific choice of antipsychotic be chosen based on a person's preference and the drug's side effect profile.[18][19][20] The U.S. Agency for Healthcare Research and Quality concludes that olanzapine is not different from haloperidol in the treatment of positive symptoms and general psychopathology, or in overall assessment, but that it is superior for the treatment of negative and depressive symptoms.[21] It has a lower risk of causing movement disorders than typical antipsychotics.[12]
In a 2013 comparison of 15 antipsychotic drugs in schizophrenia, olanzapine was ranked third in efficacy. It was 5% more effective than risperidone (4th), 24-27% more effective than haloperidol, quetiapine, and aripiprazole, and 33% less effective than clozapine (1st).[12] A 2013 review of first episode schizophrenia concluded that olanzapine is superior to haloperidol in providing a lower discontinuation rate, and in short-term symptom reduction, response rate, negative symptoms, depression, cognitive function, discontinuation due to poor efficacy, and long-term relapse, but not in positive symptoms or on the Clinical Global Impressions score. In contrast, pooled second generation antipsychotics showed superiority to first generation antipsychotics only against the discontinuation, negative symptoms (with a much larger effect seen among industry- compared to government-sponsored studies), and cognition scores. Olanzapine caused less extrapyramidal side effects, less akathisia, but caused significantly more weight gain, serum cholesterol increase, and triglyceride increase than haloperidol.[22] A 2012 review concluded that among 10 atypical antipsychotics, only clozapine, olanzapine, and risperidone were better than first generation antipsychotics.[23] A 2011 review concluded that neither first- nor second generation antipsychotics produce clinically meaningful changes in Clinical Global Impression scores but found that olanzapine and amisulpride produce larger effects on the PANSS and BPRS batteries than five other second generation antipsychotics or pooled first generation antipsychotics.[24] A 2010 Cochrane systematic review found that olanzapine may have a slight advantage in effectiveness when compared to aripiprazole, quetiapine, risperidone and ziprasidone.[17]No differences in effectiveness was detected when comparing olanzapine to amisulpride and clozapine.[17]
A 2014 meta analysis of 9 published trials having minimum duration 6 months and median duration 52 weeks concluded that olanzapine, quetiapine, and risperidone had better effects on cognitive function than amisulpride and haloperidol.[25]
Bipolar disorder
Olanzapine is recommended by the National Institute of Health and Care Excellence as a first line therapy for the treatment of acute mania in bipolar disorder.[26] Other recommended first lines are haloperidol, quetiapine and risperidone.[26] It is recommended in combination with fluoxetine as a first line therapy for acute bipolar depression; and as a second line treatment by itself for the maintenance treatment of bipolar disorder.[26]
The Network for Mood and Anxiety Treatments (CANMAT) recommends olanzapine as a first line maintenance treatment in bipolar disorder and the combination of olanzapine with fluoxetine as second line treatment for bipolar depression.[27]
A 2014 meta analysis concluded that olanzapine plus fluoxetine was the most effective among nine treatments for bipolar depression included in the analysis.[28]
Other uses
Evidence does not support the use of atypical antipsychotics, including olanzapine, in eating disorders.[29]
Olanzapine has not been rigorously evaluated in generalized anxiety disorder, panic disorder, delusional parasitosis, or post-traumatic stress disorder. Olanzapine is no less effective than lithium or valproate and more effective than placebo in treating bipolar disorder.[30] It has also been used for Tourette syndrome and stuttering.[31]
Olanzapine has been studied for the treatment of hyperactivity, aggressive behavior, and repetitive behaviors in autism.[32]
Olanzapine is frequently prescribed off-label for the treatment of insomnia, including difficult falling asleep and staying asleep. The daytime sedation experienced with olanzapine is generally comparable to quetiapine and lurasidone, which is a frequent complaint in clinical trials. In some cases, the sedation due to olanzapine impaired the ability of people to wake up at a consistent time every day. There does appear to be some evidence of efficacy for treating insomnia, but long-term studies (especially for safety) are still needed.[33]
Pregnancy and lactation
Olanzapine is associated with the highest placental exposure of any atypical antipsychotic.[34] Despite this, the available evidence suggests it is safe during pregnancy, although the evidence is insufficiently strong to say anything with a high degree of confidence.[34] Olanzapine is associated with weight gain which according to recent studies may put olanzapine-treated patients' offspring at a heightened risk for neural tube defects (e.g. spina bifida).[35][36] Breastfeeding in women taking olanzapine is advised against due to the fact that olanzapine is secreted in breast milk with one study finding that the exposure to the infant (in mg per kg of body weight, that is) is about 1.8% that to the mother.[5]
Elderly
Citing an increased risk of stroke, in 2004 the Committee on the Safety of Medicines (CSM) in the UK issued a warning that olanzapine and risperidone, both atypical antipsychotic medications, should not be given to elderly patients with dementia. In the U.S., olanzapine comes with a black box warning for increased risk of death in elderly patients. It is not approved for use in patients with dementia-related psychosis.[37]However, a BBC investigation in June 2008 found that this advice was being widely ignored by British doctors.[38] Evidence suggested that elderly are more likely to experience weight gain on olanzapine compared to aripiprazole and risperidone.[39]
Adverse effects
The principal side effect of olanzapine is weight gain, which may be profound in some cases and/or associated with derangement in the blood lipid and blood sugar profiles (see section metabolic effects). A recent meta-analysis of the efficacy and tolerance of 15 antipsychotic drugs (APDs) found that it had the highest propensity for causing weight gain out of the 15 APD compared with a SMD of 0.74[12] Extrapyramidal side effects, although potentially serious, are infrequent to rare from olanzapine[40] but may include tremors and muscle rigidity.
Several patient groups are at a heightened risk of side effects from olanzapine and antipsychotics in general. Olanzapine may produce non-trivial high blood sugar in people with diabetes mellitus. Likewise, the elderly are at a greater risk of falls and accidental injury. Young males appear to be at heightened risk of dystonic reactions, although these are relatively rare with olanzapine. Most antipsychotics, including olanzapine, may disrupt the body's natural thermoregulatory systems, thus permitting excursions to dangerous levels when situations (exposure to heat, strenuous exercise) occur.[5][6][41][42][43]
Other side effects include galactorrhea, amenorrhea, gynecomastia, and erectile dysfunction (impotence).[44]
Paradoxical effects
Olanzapine is used therapeutically to treat serious mental illness. Occasionally, it can have the opposite effect and provoke serious paradoxical reactions in a small subgroup of people, causing unusual changes in personality, thoughts, or behavior; hallucinations and excessive thoughts about suicide have also been linked to olanzapine use.[45]
Metabolic effects
The US Food and Drug Administration requires all atypical antipsychotics to include a warning about the risk of developing hyperglycemia and diabetes, both of which are factors in the metabolic syndrome. These effects may be related to the drugs' ability to induce weight gain, although there are some reports of metabolic changes in the absence of weight gain.[46][47] Studies have indicated that olanzapine carries a greater risk of causing and exacerbating diabetes than another commonly prescribed atypical antipsychotic, Risperidone. Of all the atypical antipsychotics, olanzapine is one of the most likely to induce weight gain based on various measures.[48][49][50][51][52] The effect is dose dependent in humans[53]and animal models of olanzapine-induced metabolic side effects. There are some case reports of olanzapine-induced diabetic ketoacidosis.[54]Olanzapine may decrease insulin sensitivity,[55][56] though one 3-week study seems to refute this.[57] It may also increase triglyceride levels.[49]
Despite weight gain, a large multi-center randomized National Institute of Mental Health study found that olanzapine was better at controlling symptoms because patients were more likely to remain on olanzapine than the other drugs.[58] One small, open-label, non-randomized study suggests that taking olanzapine by orally dissolving tablets may induce less weight gain,[59] but this has not been substantiated in a blinded experimental setting.
Post-injection delirium/sedation syndrome
Post-injection delirium/sedation syndrome (PDSS) is an rare syndrome that is specific to the long-acting injectable formulation of olanzapine, olanzapine pamoate.[60] The incidence of PDSS with olanzapine pamoate is estimated to be 0.07% of administrations, and is unique among other second-generation, long-acting antipsychotics (e.g. paliperidone palmitate), which don't appear to carry the same risk.[60] PDSS is characterized by symptoms of delirium (e.g. confusion, difficulty speaking, and uncoordinated movements) and sedation.[60] While not all people with PDSS will exhibit both delirium and sedation, most of them will (83%).[60] Although less specific to PDSS, a majority of cases (67%) involved a feeling of general discomfort.[60] It is thought that PDSS may occur due to accidental injection and absorption of olanzapine pamoate into the bloodstream, where it can act more rapidly, as opposed to slowly distributing out from muscle tissue.[60] Utilizing the proper, intramuscular injection technique for olanzapine pamoate helps to decrease the risk of PDSS, though it does not eliminate the risk entirely.[60] This is why the FDA advises that people that are injected with olanzapine pamoate be watched for 3 hours after administration, in the event that PDSS occurs.[60]
Animal toxicology
Olanzapine has demonstrated carcinogenic effects in multiple studies when exposed chronically to female mice and rats, but not male mice and rats. The tumors found were in either the liver or mammary glands of the animals.[61]
Discontinuation
The British National Formulary recommends a gradual withdrawal when discontinuing anti-psychotic treatment to avoid acute withdrawal syndrome or rapid relapse.[62] Due to compensatory changes at dopamine, serotonin, adrenergic and histamine receptor sites in the central nervous system, withdrawal symptoms can occur during abrupt or over-rapid reduction in dosage. However, despite increasing demand for safe and effective antipsychotic withdrawal protocols or dose-reduction schedules, no specific guidelines with proven safety and efficacy are currently available. Support groups such as the Icarus Project, and other online forums provide resources and social support for those attempting to discontinue antipsychotics and other psychiatric medications.[63] Some have argued additional somatic and psychiatric symptoms associated with dopaminergic hypersensitivity, including dyskinesia and acute psychosis, are common features of withdrawal in individuals treated with neuroleptics.[64] Thus, some suggest the withdrawal process itself may be schizo-mimetic, producing schizophrenia-like symptoms even in previously healthy patients.[65]
Overdose
Symptoms of an overdose include tachycardia, agitation, dysarthria, decreased consciousness, and coma. Death has been reported after an acute overdose of 450 mg, but also survival after an acute overdose of 2000 mg.[66] Fatalities generally have occurred with olanzapine plasma concentrations greater than 1000 ng/mL post-mortem, with concentrations up to 5200 ng/mL recorded (though this might represent confounding by dead tissue, which may release olanzapine into the blood upon death).[67] There is no known specific antidote for olanzapine overdose, and even physicians are recommended to call a certified poison control center for information on the treatment of such a case.[66] Olanzapine is considered moderately toxic in overdose, more toxic than quetiapine, aripiprazole, and the SSRIs and less toxic than the MAOIs and TCAs.[34]
注:药品如有新包装,以新包装为准。以上资讯来源于网络或由高等医药院校的学生志愿者翻译(如有错漏,请帮忙指正),仅供医护人员内部讨论,不作任何用药依据,具体用药指引,请咨询主治医师。
如您发现本网站有文字编辑或内容错误,请点击此处发送(需要安装有foxmail或outlook支持),
或发邮件至:info@pidrug.com,香港济民药业感谢您的到访!
欢迎您添加香港济民药业微信,或在公众号内留言。



